The Enthalpy Change for the Following Reaction Is
The internal energy change for a reaction is related to enthalpy by equation ΔE ΔH - PΔV. 10 Is enthalpy change of combustion negative.
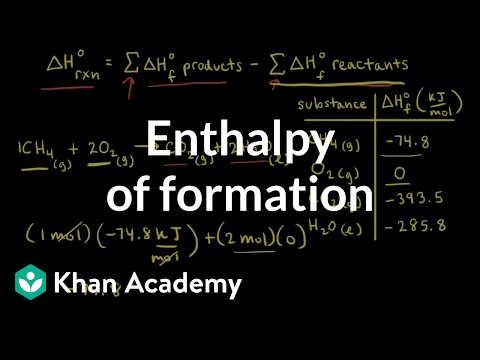
Enthalpy Of Formation Video Khan Academy
Consider the following equation where calcium oxide reacts with water.

. The enthalpy change for the following reaction is 664 kJ. Using bond energies estimate the NO bond energy in NO2g. See the answer See the answer done loading.
The enthalpy change of the following reaction CHX4 gClX2 g CHX3ClX gHClX g ΔH 104 kJ. The average X e F bond enthalpy is 3 4 k c a l m o l first ionization enthalpy of X e is 2 7 9 k c a l m o l electron gain enthalpy of flourine is 8 5 k c a l m o l and bond dissociation enthalpy of F 2 is 3 8 k c a l m o l. When estimating the enthalpy of a reaction given the standard enthalpies of formation we can apply Hess Law.
In any general chemical reaction the reactants undergo chemical changes and combine to give products. If you know these quantities use the following formula to work out the overall change. Improve your grades with step-by-step help test prep and your own study planner.
See the answer See the answer done loading. The enthalpy change for the following reaction is -4836 kJ. The most basic way to calculate enthalpy change uses the enthalpy of the products and the reactants.
234 x 105 C. H H products H reactants. The enthalpy change for the following reaction is.
Ad Find Chemistry Lessons That Cover Everything Youll Be Taught in Class Plus Much More. The enthalpy change for the following reaction is -108 kJ. We know that to calculate Enthalpy change the formula is Bond energy of H-H bond Br-Br bond 2 bond energy of H-Br In our case we can calculate enthalpy change by substituting the values.
The enthalpy of a reaction at 2 7 3 K. 4H2Og - 4H2g 2029 Select one. Using bond energies estimate the NO bond energy in NO2g.
5 7 K J. 2H2gO2g - 2H2Og therefore the enthalpy change of the following reaction is _____. 4H2 g 2O2 g 4H2O g A.
Mar 03 2019 Measuring the Enthalpy Change for a Reaction Experimentally Calorimetric method For a reaction in solution we use the following equation energy change mass of solution x heat capacity x temperature change Q J m g x cp J g-1K-1 x T K This equation will only give the energy for the actual quantities used. The reaction enthalpy is calculated by subtracting the sum of enthalpies of all the reactants from that of the products. Calculate C Cl bond enthalpy.
X e F 4 X e F F 2 F. Changing the temperature and concentration can change the initial and final state of a reaction which can affect the enthalpy of reaction. The enthalpy change of the following reaction is -4836 kJ.
Calculate C Cl bond enthalpy. CO g Cl2 g-COCl2 g Show transcribed image text. 2H2 g O2 g 2H2O g Therefore the enthalpy change for the following reaction is ________ kJ.
9 When enthalpy changes with a chemical reaction is positive then. 2H₂ g O₂ g 2H₂O g Therefore the enthalpy change for the following reaction is _____ kJ. Calculate the enthalpy change in k c a l for the reaction.
Enthalpy is a function of state. In which of the following situations would ΔH give a good approximation of ΔE. 2H2g O2g --- 2H2Og Therefore the enthalpy change for the following reaction is kJ.
This equation tells us that ΔH is very close to ΔE if there is no volume change in the reaction. 11 How is enthalpy used to predict whether a reaction is endothermic or exothermic. The enthalpy change for the following reaction is -4836 kJ.
For any such reaction the change in enthalpy is represented as Δ r H and is termed as the reaction enthalpy. The enthalpy change for the following reaction is -4836 kJ. Using bond energies estimate the NO bond energy in NO2 g.
13 What does negative enthalpy mean. ΔH ΣΔH f products ΣΔH f reactants ΔH 415 3594 556xkJ. 4H₂ g 2O₂ g 4H₂O g A -4836 B -9672 C 234 105 D 4836 E 9672.
It can be represented by the following equation. Calculate C Cl bond enthalpy. Science Chemistry QA Library The enthalpy change for the following reaction is 664 kJ.
Using bond energies estimate the CO bond energy in COCl2 g. The enthalpy change depends only on the start and end conditions of the reaction. The enthalpy change of reaction H ϴ r is the enthalpy change when reactants react to make products as stated in the chemical equation under standard conditions and in their standard states.
Ol are -942 and 61Kcalmol 1 respectivelyHeat of atomisation of carbon and hydrogen are 150 and 515Kcalmol 1 respectively. 14 Is negative enthalpy favorable. 2H2 g O2 g 2H2O g Therefore the enthalpy change - Answered by a verified Tutor We use cookies to give you the best possible experience on our website.
12 How do you determine the enthalpy of a reaction. ΔH 104 kJ. Given the following reactions Fe2O3 s 3CO s 2Fe s 3CO2 g ΔH -280 kJ 3Fe s 4CO2 s 4CO g Fe3O4 s ΔH 125 kJ the enthalpy of the reaction of Fe2O3 with CO 3Fe2O3 s CO g CO2 g 2 Fe3O4 s is ____ kJ.
See the answer See the answer done loading. -109 kJ mol-1. N2 g 2O2 g2NO2 g kJmol.
Up to 25 cash back The enthalpy change for the following reaction is -4836 kJ. -4836 B -9672 C. The bond enthalpies are - Chemistry.

5 3 1 Determine The Enthalpy Change Of A Reaction Using Hess Law Youtube

7 9701 W19 Qp 11 Hess Law Formula Energetics Mega Lecture Youtube
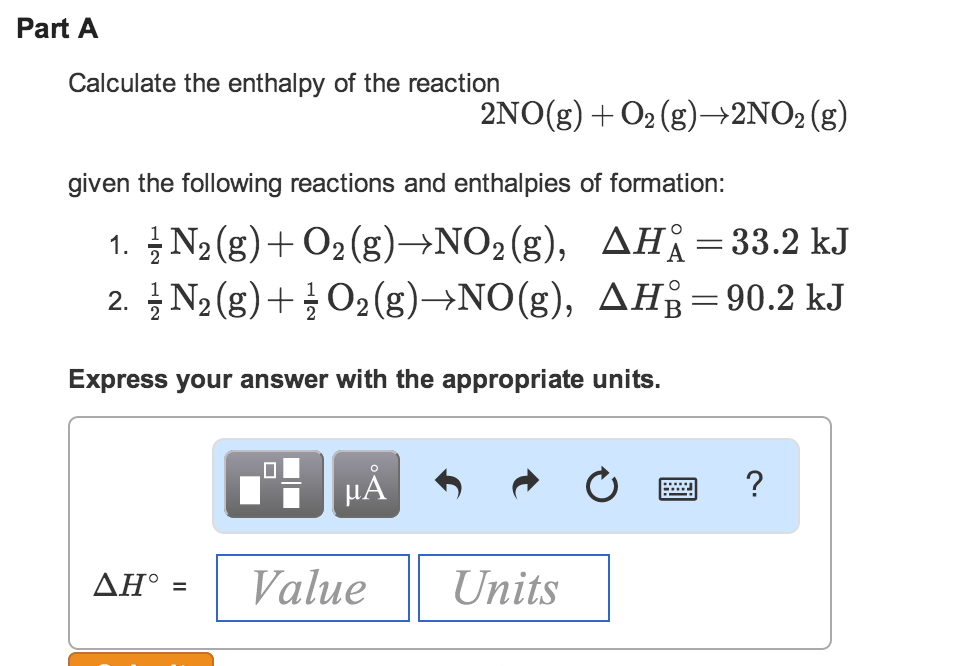
Solved Calculate The Enthalpy Of The Reaction 2n0 G Chegg Com

The Value Of Enthalpy Change D H For The Reaction C2h5oh L 3o2 G 2co2 G 3h2o L At 27 0c Is 1366 5 Kj Mol 1 The Value Of Internal Energy

Enthalpy Changes Chapter 6 Standards 5 1 5 2 5 3 And Ppt Download

Solved The Standard Enthalpy Of Formation Of Sih4 G Is 34 3 Kj The Standard Enthalpy Of Formation Reaction Is Below Si S 2 Hz G Sih4 G What Is The Enthalpy Change For The Following Reaction
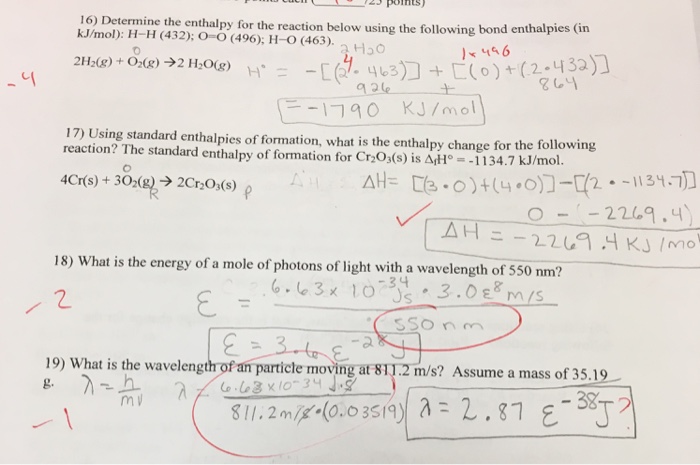
Solved Determine The Enthalpy For The Reaction Below Using Chegg Com

The Enthalpy Changes For The Following Processes Are Listed Below Cl2 G 2cl G Dh O 242 3kjmol 1 I2 G 2i G Dh O 151 0kjmol 1 Icl G I G Cl G Dh O 211 3kjmol 1 I2 S I2 G Dh O 62 76kjmol 1 Given That The Standard
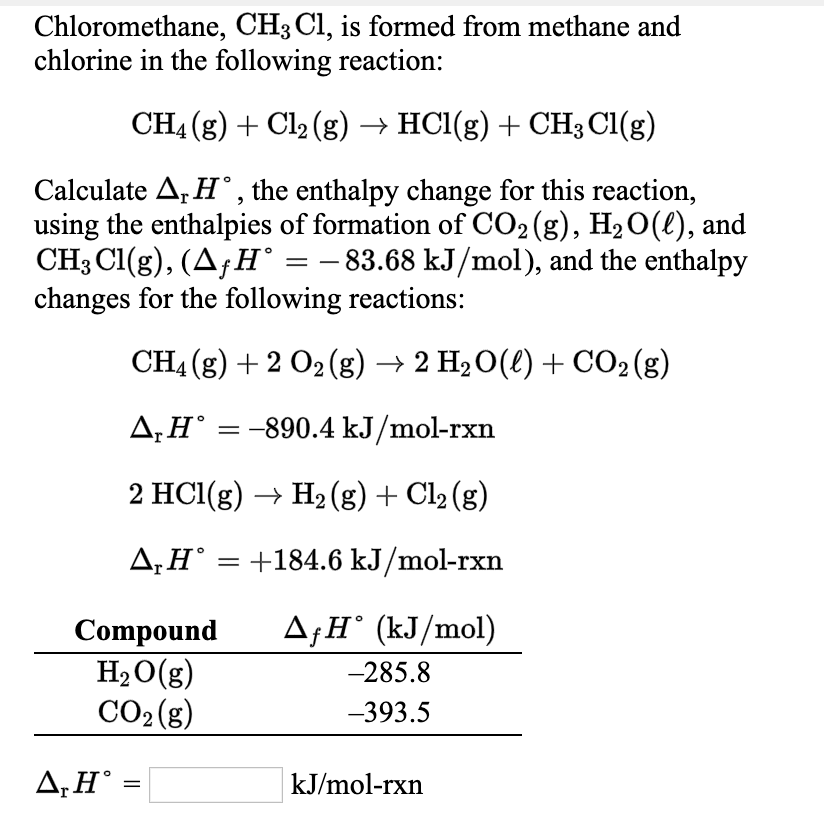
Solved Chloromethane Ch3 Cl Is Formed From Methane And Chegg Com

Calculate The Standard Enthalpy Of The Reaction From The Following Dh Values Chemistry Shaalaa Com

The Enthalpy Change For Which Of The Following Processes Represents The Enthalpy Of Formation Of Agcl
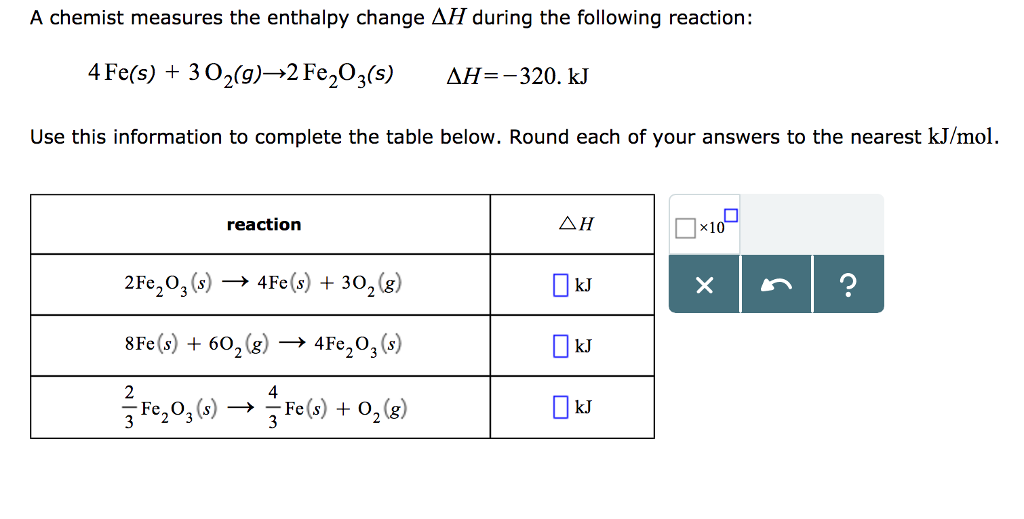
Solved A Chemist Measures The Enthalpy Change Dh Chegg Com

5 1 Standard Enthalpy Changes Of Formation And Combustion Youtube

The Enthalpy Change For The Reaction N2 G 3h2 G 2nh3 G 92 2kj Mol Youtube

Use The Given Standard Enthalpies Of Formation In Kj Mol To Determine The Enthalpy Of Reaction Of The Following Reaction Nh3 G 3f2 G Nf3 G 3hf G Dhf Nh3 G 46 2 Dhf Nf3 G

Lesson 6 Hess Law And Enthalpy Cycles Using Enthalpy Of Combustion Ppt Download

Lesson 6 Hess Law And Enthalpy Cycles Using Enthalpy Of Combustion Ppt Download

Comments
Post a Comment